Issued on behalf of Oncolytics Biotech Inc.
With Phase 2 clinical results showing an estimated 76% survival improvement in HR+/HER2- metastatic breast cancer and a pancreatic cancer study cohort funded by PanCAN, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is capturing attention with a $4.79 price target[1]—an over 487% upside from its current valuation!
Cancer—a word that strikes fear into the hearts of many. Yet a revolution in how this formidable foe is fought is unfolding.
In recent years, cancer rates among younger generations—particularly Generation X and millennials—have risen sharply, rewriting our understanding of health in modern society. [2] A complex web of lifestyle changes[3], environmental shifts[4], and genetic predispositions[5] has fueled this troubling trend. The result? A growing burden on families, healthcare systems, and economies worldwide.
The stakes have never been higher. The global economic cost of cancer is projected to exceed $25 trillion over the next three decades[6]. Traditional treatments like chemotherapy and radiation, while life-saving for millions, are reaching their limits, leaving patients and physicians alike searching for more effective and less taxing options.
The demand for innovative solutions has created a booming market:
- The global cancer therapeutics market is expected to grow from $98.9 billion in 2018 to $180.2 billion by 2026 at a 7.7% CAGR. [7]
- The breast cancer drug market alone is projected to reach $55.9 billion by 2030, growing at an 8.2% CAGR. [8]
- Pancreatic cancer treatments, one of the most challenging areas in oncology, are anticipated to expand at a 4% CAGR, reaching $10.2 billion by 2034. [9]
Big Pharma has taken notice. Companies like Roche, AstraZeneca, and Pfizer are investing billions in partnerships and acquisitions, signaling a shift toward targeted immunotherapies and next-generation approaches. These companies know the future of cancer treatment isn’t just about attacking tumors—it’s about empowering the body’s own defenses to fight back. [10],[11],[12],[13],[14],[15]
Imagine treatments that don’t just kill cancer cells but teach the immune system how to prevent them from coming back. This isn’t a distant dream; it’s happening now. A new wave of therapies is poised to transform the way cancer is fought.
But among the countless innovations in oncology, one therapy is capturing the attention of scientists, physicians, and analysts alike—a groundbreaking approach that could redefine cancer care as we know it.
Enter pelareorep—a groundbreaking therapy that could one day redefine cancer treatment as we know it.
This innovative immunotherapy is making waves in the medical community by leveraging the body’s immune system to target and destroy cancer cells.
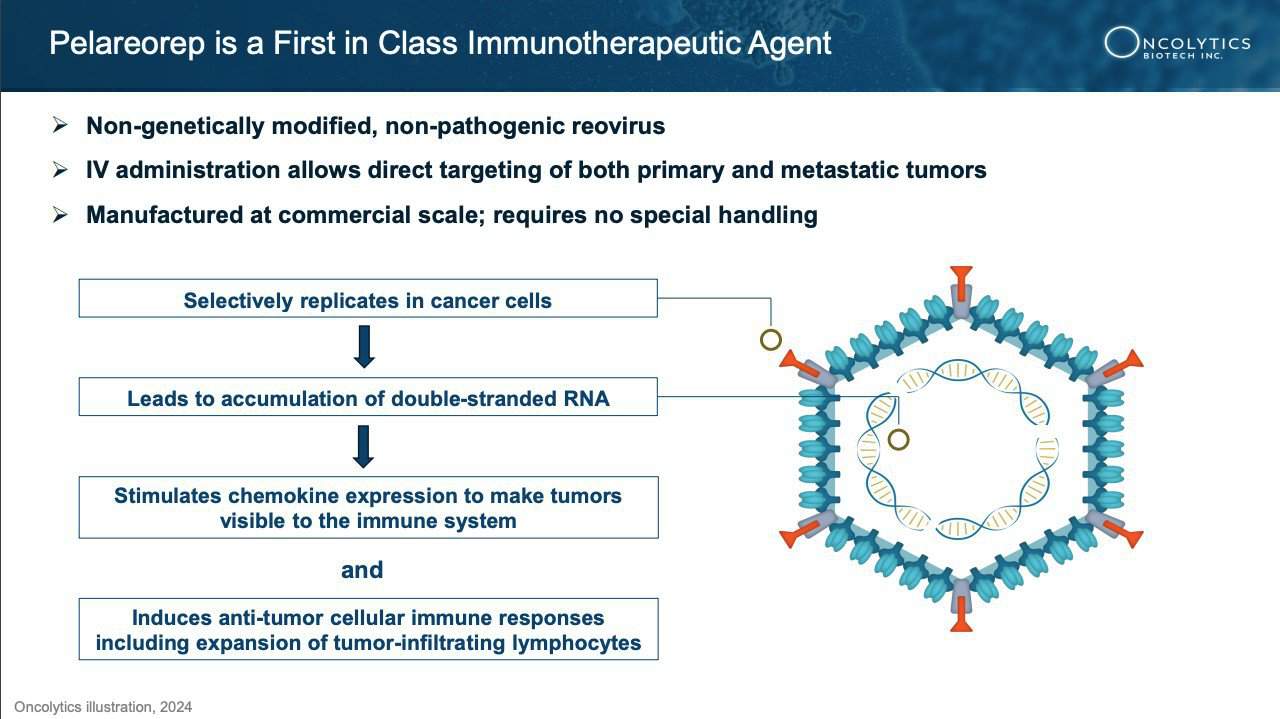
Recent trials have shown promising results, with significant clinical benefits, including improvements in survival rates for patients with some of the most challenging cancers.[16],[17],[18]
The driving force behind this revolutionary treatment is Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC), a pioneering company at the forefront of immunotherapy innovation.
Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC)
With a commitment to improving the quality of life for cancer patients, Oncolytics is harnessing the potential of pelareorep to unlock the body’s antitumor capabilities.
In clinical studies, pelareorep has demonstrated remarkable success, particularly in metastatic breast cancer and pancreatic cancer. Patients treated with pelareorep and standard chemotherapy in the BRACELET-1 trial saw a 76% estimated improvement in overall survival, while the GOBLET trial showed a 62% objective response rate in pancreatic cancer. These breakthroughs have not gone unnoticed.
Backed by collaborations with industry giants like Roche, Pfizer, and Merck, as well as funding from the Pancreatic Cancer Action Network (PanCAN), Oncolytics is advancing pelareorep to late-stage clinical trials. With both breast cancer and pancreatic cancer receiving FDA Fast Track Designation, the path to regulatory approval is being accelerated, offering new hope to millions of patients worldwide.
But how does this innovative treatment work? And what does it mean for the future of cancer care? Stay tuned as we delve deeper into the science behind pelareorep and its potential to transform cancer treatment.

The Top 7 Reasons
Oncolytics Biotech Inc.
(NASDAQ:ONCY) (TSX:ONC)
is a Name to Watch in Biotech
1 Regulatory Triumphs: Oncolytics Biotech is making significant strides with TWO FDA Fast Track Designations [19],[20] for pelareorep in metastatic breast and pancreatic cancer. This designation allows for greater collaboration with the FDA in addition to potential expedited review and approval as Oncolytics prepares for a pivotal trial in HR+/HER2- metastatic breast cancer in 2025[21].
2 Strategic Alliances: Oncolytics is collaborating with industry leaders like Pfizer, Roche, and Merck, [22] and has secured funding from the Pancreatic Cancer Action Network (PanCAN) to advance its pancreatic cancer program. These alliances enhance pelareorep’s development across multiple indications and validate its promise.
3 Clinical Breakthroughs: In recent trials, pelareorep demonstrated a 76% estimated improvement in overall survival (OS) for metastatic breast cancer patients in combination with chemotherapy (32.1 months vs. 18.2 months) [23]. Additionally, in pancreatic cancer, the GOBLET trial revealed a promising 62% objective response rate (ORR) when combined with checkpoint inhibitor monotherapy, and was recently given regulatory approval to continue patient enrollment into Cohort 5 of the study, which evaluates pelareorep with modified FOLFIRINOX (mFOLFIRINOX) with or without atezolizumab (Tecentriq®)[24].
4 Market Expansion: With the global oncology market projected to grow to $180.2 billion by 2026 (7.7% CAGR) [25], and breast and pancreatic cancer treatments poised for significant expansion, Oncolytics is positioned to capture substantial market share with its innovative immunotherapeutic agent.
5 Expert Leadership: Led by a team of seasoned professionals, Oncolytics Biotech boasts decades of experience in drug development and commercialization. Their expertise is guiding the company through pivotal clinical milestones and strategic growth.
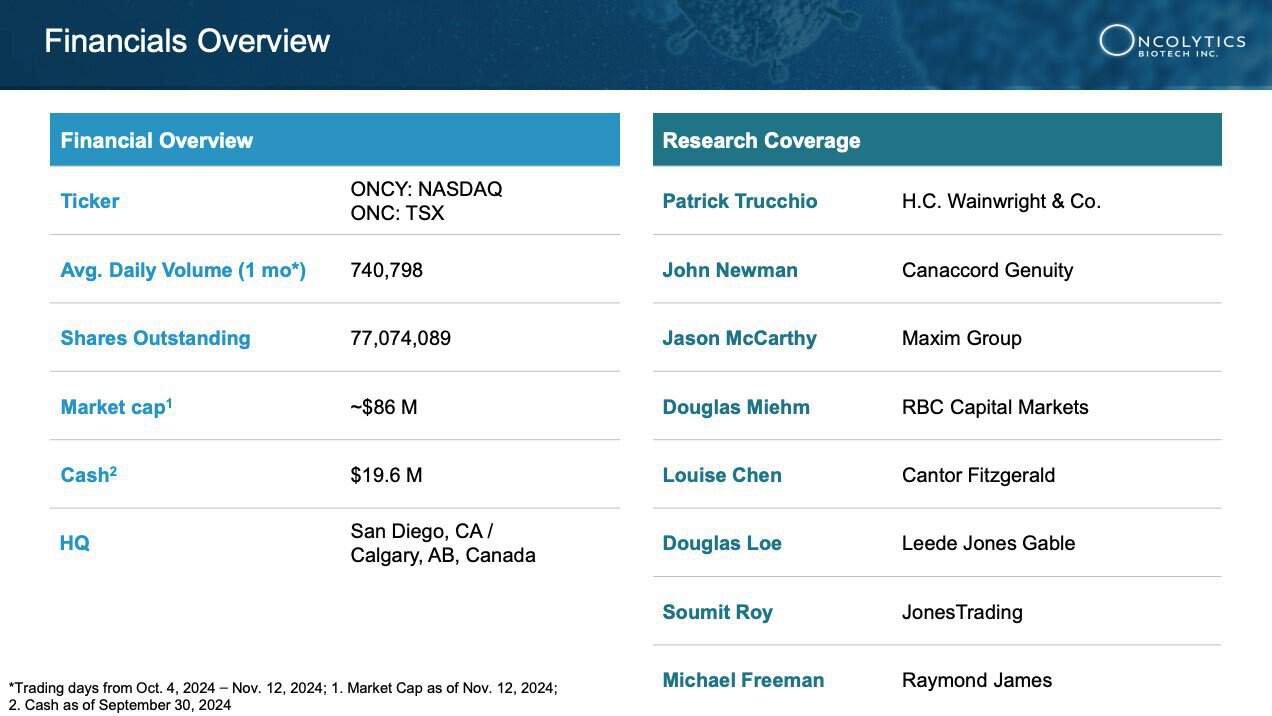
6 Robust Financial Health: With a cash position of $19.6 million, Oncolytics is well-equipped to fund ongoing research and key milestones into 2025, including additional data readouts and what is believes is a clear pathway to future commercialization opportunities. [26]
7 Commitment to Innovation: Oncolytics remains steadfast in its mission to advance cancer research. By turning “cold” tumors into “hot” ones that respond better to treatment, pelareorep is addressing critical gaps in cancer immunotherapy.
WHAT Analysts Are Saying + WHY the Market Is Slow to Catch On
Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is currently trading at $0.82, but analysts project a 1-year price target of $4.79, reflecting a potential upside of over 4870%. According to Yahoo! Finance (as of January 17, 2025)[27], Oncolytics boasts 8 Buy ratings, including 5 Strong Buys, underscoring strong confidence in the company’s future.
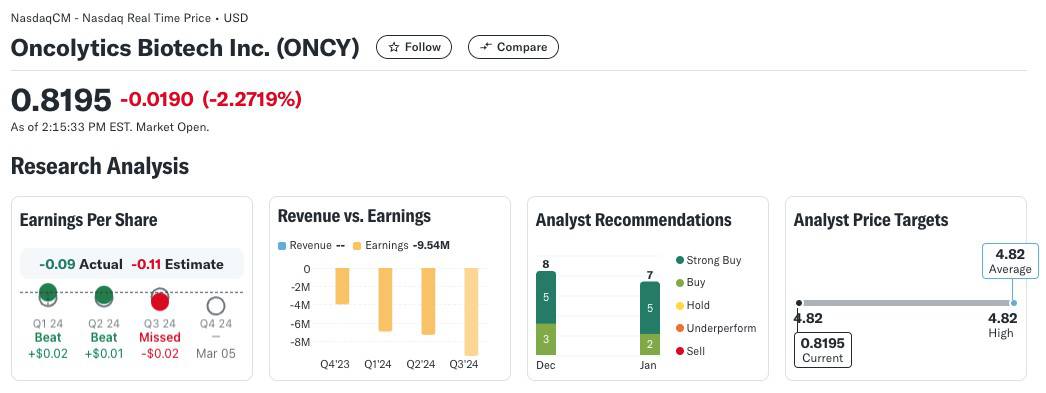
Despite the company’s remarkable Phase 2 BRACELET-1 results, which showed a 76% estimated improvement in overall survival for metastatic breast cancer patients, the market has been slow to respond. Why?
Historically, smaller biotech companies often see delayed stock reactions to groundbreaking data. It’s not uncommon for the big jumps in share price to come after pivotal milestones like:
- Regulatory approvals
- Key Phase 3 trial data
- Partnership announcements with major pharmaceutical players
For Oncolytics, the stage is set for significant catalysts like these in the future. With preparations underway for a registration-enabling study in metastatic breast cancer and advancements in its pancreatic cancer program supported by PanCAN funding, the pieces are falling into place.
This lag in market reaction creates a rare window for investors. The analysts see it. The data supports it. And the company’s momentum is building. The market hasn’t yet priced in the full potential of Oncolytics Biotech—and that’s where savvy investors can capitalize.
Don’t wait until the crowd catches on. Now is the time to act.
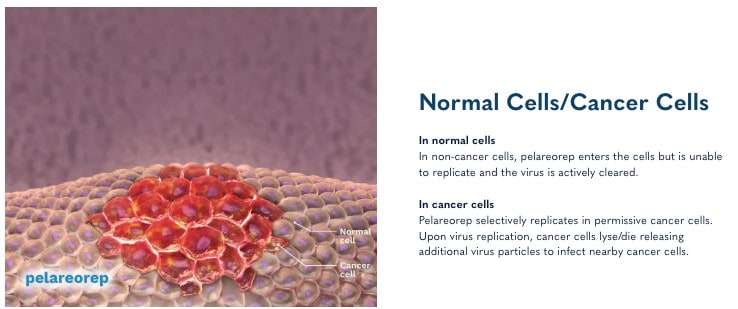
Pelareorep: Mechanism and Potential
Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is a trailblazer in cancer treatment, harnessing the power of the body’s immune system with pelareorep to target and eliminate cancer cells. This immunotherapeutic agent works by enhancing the effectiveness of chemotherapy and immunotherapy, transforming the tumor microenvironment to be more hostile to cancer.
How does it do this? Pelareorep transforms the tumor microenvironment by generating and recruiting immune cells to recognize and attack cancer. It enhances immune response by introducing double-stranded RNA into cancer cells, leading to inflammation and upregulation of PD-L1, which helps T-cells infiltrate and destroy the tumor.
This innovative approach allows pelareorep to work in harmony with chemotherapy, immune checkpoint inhibitors, and other therapies, boosting their effectiveness and offering hope for longer-lasting remissions.
Research and development efforts are in full swing, with key clinical trials like BRACELET-1 for breast cancer and GOBLET for pancreatic and anal cancer leading the charge. These studies have shown impressive results, including significant improvements in objective response rate, progression-free survival, and overall survival rates.
Pelareorep’s potential is expansive, offering hope across multiple cancer types by converting “cold” tumors into “hot” ones, making them more susceptible to attack from the immune system. It’s not just another cancer treatment—it’s a revolutionary approach to unleashing the full power of the immune system.

Transforming Breast Cancer Treatment
Breast cancer remains a formidable challenge, with over 310,000 new cases estimated to be diagnosed in the U.S. in 2024.[28] But with Oncolytics Biotech Inc.’s (NASDAQ:ONCY) (TSX:ONC) pelareorep, there’s new hope. It’s already showing big improvements for patients with HR+/HER2- metastatic breast cancer, especially those who have exhausted other treatment options.
With annual expenses reaching a total of $29.8 billion[29] for the total breast cancer market, HR+/HER2- metastatic breast cancer patients often face limited options once earlier-stage treatments like hormonal therapy or later-stage treatments like antibody-drug conjugates fail, leaving a significant unmet need in treatment.[30]
Recently, Oncolytics reported favorable positive clinical results from its randomized Phase 2 study, BRACELET-1,[31] reinforcing their path towards a registration-enabling study as a result of constructive dialog with the FDA. [32]
A registration-enabling study is a pivotal clinical trial that is designed to provide the necessary data for a New Drug Application (NDA) submission to the FDA for approval.
Within the latest BRACELET-1 results, pelareorep (when combined with chemotherapy) helped patients go 12.1 months before their cancer got worse (median PFS)—almost double the time for patients on chemotherapy alone.[33]
Even better, the median overall survival (OS) could not be determined, as more than half of the patients were still alive at the study’s conclusion. However, estimates put OS at approximately 32 months compared to 18.2 months for chemotherapy alone.
This data builds on the strong findings from the earlier IND-213 study, which also demonstrated a significant survival benefit when combining pelareorep with chemotherapy. Given this consistent success, Oncolytics Biotech is seemingly advancing pelareorep toward a registration-enabling trial[34].
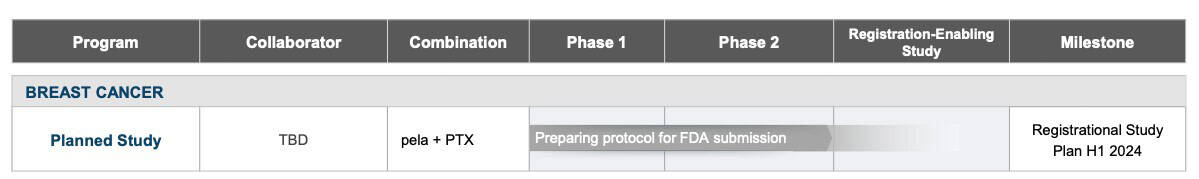
FDA Process and Market Potential
Back in 2017, the FDA awarded pelareorep with Fast Track Designation for the treatment of metastatic breast cancer [35], providing a potential expedited regulatory review process and potential for accelerated approval.
How significant would a potential approval be?
The breast cancer drug market is poised for rapid growth, with projections from BioSpace showing it will grow from $32.93 billion in 2023 to $78.61 billion by 2033[36]. Additionally, DelveInsight predicts a 9.3% CAGR for the HR+/HER2- metastatic breast cancer market alone[37], underscoring the significant commercial opportunity for new treatments.
With promising results and regulatory momentum, pelareorep is poised to make a significant impact in the fight against breast cancer, offering new hope to patients worldwide.

Tackling Pancreatic Cancer
Pancreatic cancer. It’s known as the “silent killer,” and for a good reason. Each year, about 66,440 people in the U.S. are expected to be diagnosed[38], and it’s one of the deadliest cancers, with a five-year survival rate of just 13%.[39]
The symptoms are subtle, often going unnoticed until it’s too late, and the incidence rate among young women has skyrocketed by 208% over the past 30 years.[40]
Enter pelareorep, a game-changing immunotherapy with the potential to turn the tide. In clinical trials, pelareorep has been making waves, especially when combined with treatments like chemotherapy and immune checkpoint inhibitors.
Recent data from the GOBLET study revealed a remarkable 62% objective response rate,[41] more than doubling the results of historical control trials.[42]
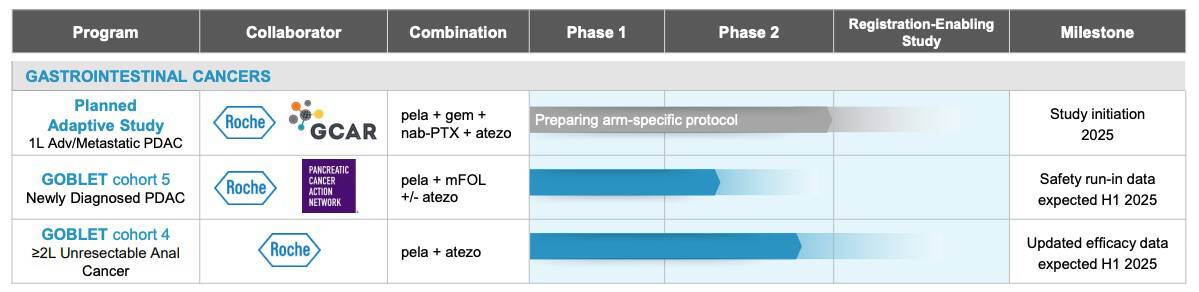
This promising progress hasn’t gone unnoticed. The Pancreatic Cancer Action Network (PanCAN) awarded Oncolytics Biotech a US$5 million Therapeutic Accelerator Award to support the continued development of pelareorep.[43]
The FDA has also taken notice, granting pelareorep Fast Track Designation, which is designed to improve dialog with regulators and potentially accelerating its journey toward potential approval.[44] In January, Germany’s medical regulatory body, the Paul-Ehrlich-Institute (PEI) approved the continuation of patient enrollment into Cohort 5 of the GOBLET study. This cohort is evaluating pelareorep in combination with modified FOLFIRINOX (mFOLFIRINOX) with or without atezolizumab (Tecentriq®) in newly diagnosed pancreatic ductal adenocarcinoma (PDAC) patients.[45]
Backed by robust collaborations and cutting-edge science, pelareorep is not just making headlines—it’s potentially poised to set new standards in pancreatic cancer treatment. With each success, pelareorep is proving that the future of cancer treatment is nearly here, and it could be brighter than ever.

Advancing Solutions for Anal Cancer
The number of new anal cancer cases has been rising for many years, with a rise in incidence of 2.7% annually for the past decade in the USA[46]. The number of people who die from anal cancer each year has also been rising[47].
Contributing factors include HPV infection, lowered immunity, and smoking[48]. There are treatment options for anal cancer, but they usually do not come cheaply.
In a 2018 study published in the Journal of Managed Care + Specialty Pharmacy, it was determined the average cost of the first 2 years of anal cancer treatment to be $127,531.[49]
The American Cancer Society estimates about 10,540 new cases of anal cancer in 2024 (3,360 in men, and 7,180 in women), with about 2,190 deaths (1,000 in men, and 1,190 in women). [50]
In response to this growing concern, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is positioning pelareorep towards the forefront of innovative treatment strategies. This cutting-edge therapy is being integrated into a combination therapy and is currently being tested in a clinical trial alongside an immune checkpoint inhibitor.[51]
Recent trials have shown promising results, with pelareorep delivering positive interim data from the unresectable squamous cell carcinoma of the anal canal (SCCA) cohort of the Phase 2 GOBLET study, including a complete response in an indication where checkpoint inhibitor therapy alone has had limited success historically.[52] These results demonstrated an almost a 3X improvement compared to data from like studies evaluating checkpoint inhibitors in patients with more than one prior line of therapy.[53],[54],[55]
With the support of leading pharmaceutical companies and academic institutions, Oncolytics Biotech is advancing pelareorep through a strategic pathway toward potential regulatory approval. The future implications are profound, offering a beacon of hope for more effective cancer care.
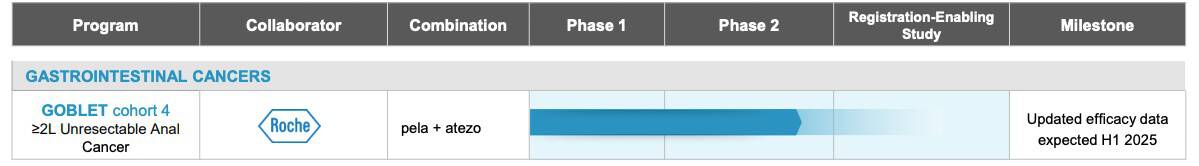
Financial Position and Market Overview
Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) stands on solid financial ground, boasting a cash position of $19.6 million (as of Sept. 30, 2024)[56], which provides a financial runway through key milestones well into 2025.
The company’s share structure is designed to support its growth strategy, ensuring stability as Oncolytics continues to push the boundaries of immunotherapy.
AS MENTIONED ABOVE: Analysts consistently recognize Oncolytics’ strong market position and potential for substantial growth, with 4 analysts offering a consensus STRONG BUY rating.[57]
Recent strategic collaborations, such as the partnership with the Global Coalition for Adaptive Research (GCAR)[58] and funding from PanCAN,[59] underscore the company’s capability to secure critical resources and partnerships. These alliances not only bolster Oncolytics’ financial standing but also accelerate its path toward revolutionizing cancer treatment with pelareorep.
With a strategic financial framework and an innovative pipeline, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is poised for growth, paving the way for groundbreaking advancements in oncology.
ONCY Among Its Peers
Now let’s look at how Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) stacks up to its peers as a potential takeout target in the future by pharma giants looking for an oncology winner:
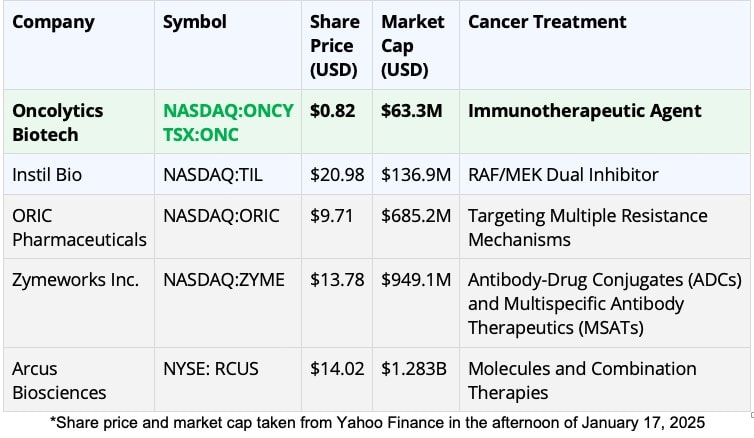
It’s clear that in the world of cancer treatment, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) stands out in this busy field.

MEET THE EXPERTS AT ONCOLYTICS BIOTECH
Developing a revolutionary treatment like pelareorep requires a powerhouse team, and Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) has just the right lineup to deliver. With experience from industry giants like Amgen, Bristol-Myers Squibb, and academic institutions such as Harvard Medical School, Oncolytics’ team is second to none.
Currently leading the charge is Wayne Pisano, Interim CEO and Chair of the Board, stepping in for Dr. Matt Coffey during his medical leave. Wayne Pisano is an industry veteran with over 30 years of experience in pharmaceuticals and 12 years in biotech.
Formerly the President and CEO of Sanofi Pasteur, he played a pivotal role in making it a leader in the global influenza vaccine market.[60] Pisano’s interim leadership at Oncolytics ensures the company remains on track to achieve its strategic priorities and advance pelareorep through critical clinical milestones.
Let’s meet some of them:
Matt Coffey, PhD, MBA – Co-Founder, Director, President & CEO
A leading expert in cancer virotherapy, Dr. Coffey has dedicated his career to studying how viruses can be harnessed to combat cancer. His groundbreaking research has been published in numerous prestigious scientific journals. Currently on medical leave, his vision continues to guide Oncolytics’ mission and strategy.
Patricia S. Andrews – Director
An accomplished biopharmaceutical executive, Pat Andrews brings extensive experience (including years with Pfizer) in navigating registrational trials and completing transformative business deals. Her strategic insights are instrumental as Oncolytics advances pelareorep toward pivotal studies.
Thomas Heineman, MD, PhD – Head of Clinical Development and Operations
With over 25 years in drug development, Dr. Heineman has led numerous oncology projects, focusing on advancing treatments for cancers such as breast and pancreatic cancer.
Deborah M. Brown, B.Sc., MBA – Director
Known for her strategic leadership at EMD Serono and Accelera Canada, Deborah Brown is instrumental in guiding new medical businesses to success.
Bernd R. Seizinger, MD, PhD – Director
An expert in cancer drug discovery, Dr. Seizinger has served in top roles at Harvard Medical School and Princeton University, contributing to groundbreaking advancements in oncology.
Guided by these exceptional minds, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is paving the way for innovative cancer therapies that could change the lives of patients worldwide.
RECAP: 7 Things to Remember About
Oncolytics Biotech Inc.
(NASDAQ:ONCY) (TSX:ONC).
 1. Regulatory Triumphs
1. Regulatory Triumphs
2. Strategic Alliances
3. Clinical Breakthroughs
4. Market Expansion
5. Expert Leadership
6. Robust Financial Health
7. Commitment to Innovation
Join the Revolution in Cancer Care
The fight against cancer is entering a transformative era, and Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is leading the charge. With pelareorep delivering breakthrough results in metastatic breast and pancreatic cancers, the potential to save and improve millions of lives is within reach.
Backed by visionary leadership, strategic partnerships with industry giants, and a strong financial position, Oncolytics is at the forefront of innovation in oncology. This is your chance to join a company that’s redefining cancer treatment with cutting-edge immunotherapy.
Whether you’re an investor, collaborator, or supporter, there’s a place for you in this groundbreaking journey. Together, we can push the boundaries of what’s possible in cancer care and bring hope to patients worldwide.
Stay informed and be part of the movement. Sign up for email alerts to receive the latest updates on Oncolytics Biotech’s progress, key milestones, and opportunities to get involved.
The future of oncology is here—don’t miss your chance to be a part of it.
USA News Group
Editorial Staff
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by MIQ has been approved by the above mentioned company; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles.
While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
SOURCES CITED:
[1] https://www.tipranks.com/stocks/oncy/forecast
[2] https://www.today.com/health/disease/cancer-rising-millennials-study-rcna164610
[3] https://www.cityofhope.org/whats-behind-rising-colorectal-cancer-rates-in-young-adults
[4] https://abcnews.go.com/Health/cancer-deaths-declining-troubling-increase-colon-breast-cancer/story?id=106427957
[5] https://edition.cnn.com/2024/07/31/health/millennials-gen-x-cancer-risk-study/index.html
[6] https://www.healio.com/news/hematology-oncology/20230223/global-economic-cost-of-cancer-estimated-to-exceed-25-trillion-over-30-years
[7] https://www.einnews.com/pr_news/728043865/cancer-therapeutics-market-exploring-global-investment-and-r-d-trends-cagr-7-7
[8] https://www.openpr.com/news/3596177/breast-cancer-drugs-market-size-share-trends-key-drivers
[9] https://www.openpr.com/news/3585977/at-a-cagr-of-12-4-pancreatic-cancer-treatment-market
[10] https://www.reuters.com/breakingviews/super-chemo-will-be-big-pharmas-next-tussle-2022-05-16/
[11] https://www.forbes.com/sites/greatspeculations/2019/07/10/was-the-47-billion-acquisition-of-genentech-in-2009-a-good-deal-for-roche/?sh=5c78818aa1aa
[12] https://www.fiercebiotech.com/biotech/eat-me-pfizer-swallows-cd47-biotech-trillium-2-3b-takeover
[13] https://www.wsj.com/business/ftc-settles-with-amgen-over-27-8-billion-deal-for-horizon-therapeutics-b96a2d69
[14] https://ir.horizontherapeutics.com/news-releases/news-release-details/horizon-therapeutics-plc-announces-fda-approval-update
[15] https://www.fiercebiotech.com/biotech/sanofi-inks-2-5b-synthorx-takeover-to-gain-il-2-cancer-drug
[16] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-reports-favorable-results-for-bracelet-1-breast-cancer-study-reinforcing-path-to-funding-of-a-registration-enabling-study/
[17] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-to-present-promising-pelareorep-data-in-pancreatic-and-anal-cancers-at-asco-gi-symposium/
[18] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-announces-key-progress-and-upcoming-studies-for-breast-and-pancreatic-cancer-treatments-prepares-for-fda-accelerated-approval-path/
[19] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-receives-fda-fast-track-designation-for-the-treatment-of-advanced-metastatic-pancreatic-cancer/
[20] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-inc-announces-fda-fast-track-designation-for-reolysin-in-metastatic-breast-cancer/
[21] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-announces-productive-fda-type-c-meeting-on-its-metastatic-breast-cancer-program/
[22] https://oncolyticsbiotech.com/pipeline/
[23] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-advances-toward-registration-enabling-trial-for-pelareorep-in-breast-cancer-with-submission-of-type-c-meeting-request-to-fda-2/
[24] https://finance.yahoo.com/news/regulatory-approval-clears-path-oncolytics-120000409.html
[25] https://www.einnews.com/pr_news/728043865/cancer-therapeutics-market-exploring-global-investment-and-r-d-trends-cagr-7-7
[26] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-to-host-conference-call-to-discuss-third-quarter-financial-results-and-recent-operational-highlights-3/
[27] https://finance.yahoo.com/quote/ONCY/analysis/
[28] https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html
[29] https://www.cdc.gov/nccdphp/priorities/breast-cancer.html#:~:text=%2429.8%20billion%3A2-,Breast%20cancer%20has%20the%20highest%20treatment%20cost%20of%20any%20cancer,%243.5%20billion%20for%20prescription%20drugs.
[30] https://www.mdanderson.org/newsroom/sabcs-studies-suggest-novel-targeted-therapies-may-benefit-patients-metastatic-hr-plus-her2-breast-cancer.h00-159624168.html
[31] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-reports-favorable-results-for-bracelet-1-breast-cancer-study-reinforcing-path-to-funding-of-a-registration-enabling-study/
[32] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-announces-productive-fda-type-c-meeting-on-its-metastatic-breast-cancer-program/
[33] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-reports-favorable-results-for-bracelet-1-breast-cancer-study-reinforcing-path-to-funding-of-a-registration-enabling-study/
[34] https://www.onclive.com/view/fda-receives-type-c-meeting-request-for-pelareorep-in-hr-her2-metastatic-breast-cancer
[35] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-inc-announces-fda-fast-track-designation-for-reolysin-in-metastatic-breast-cancer/
[36] https://www.biospace.com/breast-cancer-drugs-market-size-to-surpass-usd-78-61-bn-by-2033
[37] https://www.prnewswire.com/news-releases/metastatic-hrher2-breast-cancer-market-is-expected-to-showcase-a-significant-growth-at-a-cagr-of-9-3-by-2032–predicts-delveinsight-302004113.html
[38] https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html
[39] https://pancan.org/news/pancreatic-cancer-five-year-survival-rate-increases-to-13/
[40] https://www.dailymail.co.uk/health/article-13033795/Huge-spike-cancer-sparks-alarm-experts-baffled.html
[41] https://oncolyticsbiotech.com/press_releases/oncolytics-presents-positive-updated-pancreatic-cancer-data-from-goblet-phase-1-2-study-at-esmo/
[42] https://www.nejm.org/doi/full/10.1056/NEJMoa1304369
[43] https://pancan.org/news/pancans-5-million-therapeutic-accelerator-award-goes-to-oncolytics-biotech-inc/
[44] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-receives-fda-fast-track-designation-for-the-treatment-of-advanced-metastatic-pancreatic-cancer/
[45] https://finance.yahoo.com/news/regulatory-approval-clears-path-oncolytics-120000409.html
[46] https://pubmed.ncbi.nlm.nih.gov/31742639/
[47] https://seer.cancer.gov/statfacts/html/anus.html
[48] https://www.cancer.org/cancer/types/anal-cancer/causes-risks-prevention/what-causes.html
[49] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10397580/
[50] https://www.cancer.org/cancer/types/anal-cancer/about/what-is-key-statistics.html
[51] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-initiates-enrollment-expansion-of-goblet-anal-cancer-cohort/
[52] https://oncolyticsbiotech.com/press_releases/oncolytics-announces-the-anal-cancer-cohort-of-the-goblet-phase-1-2-study-of-pelareorep-and-atezolizumab-has-met-the-success-criteria-for-efficacy/
[53] https://pubmed.ncbi.nlm.nih.gov/28453692/
[54] https://pubmed.ncbi.nlm.nih.gov/28223062/
[55] https://pubmed.ncbi.nlm.nih.gov/35816951/
[56] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-reports-third-quarter-2024-financial-results-and-operational-highlights/
[57] https://www.tipranks.com/stocks/oncy/forecast
[58] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-announces-preliminary-collaboration-with-gcar-for-inclusion-of-pelareorep-in-anticipated-pancreatic-cancer-trial/
[59] https://pancan.org/press-releases/the-pancreatic-cancer-action-network-selects-oncolytics-biotech-inc-to-receive-5-million-therapeutic-accelerator-award-to-develop-leading-edge-treatments/
[60] https://oncolyticsbiotech.com/press_releases/oncolytics-biotech-announces-president-and-chief-executive-officer-dr-matt-coffey-to-take-medical-leave-of-absence/

